Hello Friends,
Nutty Scientists Canada Wishing YOU and Your Family a Happy Christmas & Wonderful Holidays !!
As you know Nutty Scientists Canada, recently had a science event for our little Canadian scientists. We have received huge feedback from the participants of the event. Most of the common questions about the dry ice experiments and the science behind the dry ice. On your request, our research team like to present some information / facts on DRY ICE and we are sure you will be happy to read about DRY ICE.
Please do not forget to like our post on social media as your likes & follow will encourage our little scientists to research more scientific notions. THANK YOU !!
Below is the information on DRY ICE : Enjoy reading !!
What is Dry Ice:
Dry ice is the solid form of carbon dioxide (CO2). It's called "dry ice" because it does not melt like wet ice. Instead, dry ice converts into carbon dioxide gas.
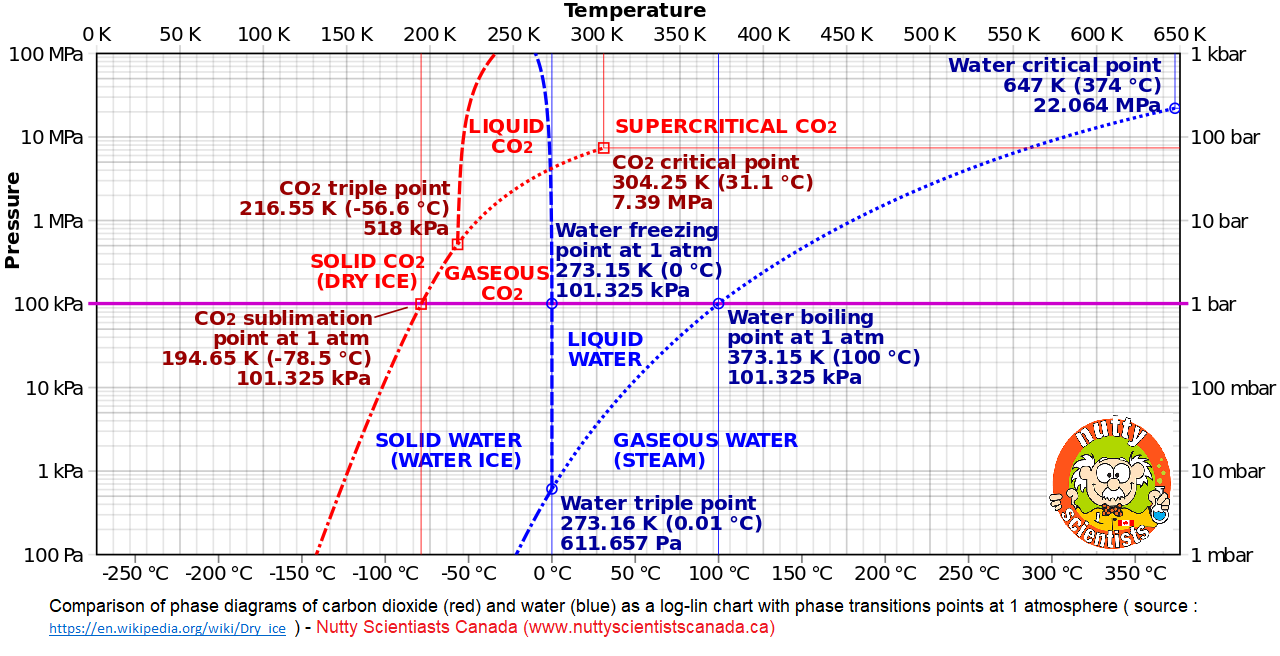
Dry Ice is extremely cold, sublimates at -78.5 C or 109.3 F at earth atmospheric pressures. A molecule consisting of a single carbon atom bonded to two oxygen atoms. Dry ice is colorless, non-flammable, with a sour zesty odor.
When Carbon Dioxide ( CO2 ) Changes from a solid to a gas with no intervening liquid form, this process called SUBLIMATION. The opposite process called DEPOSITION when CO2 changes from the gas to solid phase – DRY ICE.
When CO2 is in solid state, called as Dry Ice, it must be kept at -109 degrees. When Dry Ice is exposed to air, it converts (sublimates) back to its natural state, a gas
History about Dry Ice:
It is generally accepted that dry ice was first observed in 1835 by French inventor Adrien Jean Pierre Thilorier (1790–1844), who published the first account of the substance. In his experiments, it was noted that when opening the lid of a large cylinder containing liquid carbon dioxide, most of the liquid carbon dioxide quickly evaporated. This left only solid dry ice in the container. In 1924, Thomas B. Slate applied for a US Patent to sell dry ice commercially. Subsequently, he became the first to make dry ice successful as an industry.
How its made:
The manufacturing process of Dry Ice involves two different steps. First, pure CO2 is expanded to a reduced pressure in a hydraulic press. The CO2 "snow" that is produced in this expansion is then compressed into blocks of Dry Ice.
Dry Ice is being manufacturing in form of either as a blocks, nuggets or pellets, depend on its uses for industrial or commercial use.
Where Dry Ice being used:
Dry Ice is being use mostly in industrial and commercial use. Dry Ice is commonly used as an expendable refrigerant to ship frozen food or medical products or to cool materials during production. The other major use of dry ice is for dry ice blast cleaning, an effective and environmentally friendly way to clean industrial equipment.
Safety with handling dry ice:
Dry Ice MUST be handled with special care and protection. The Dry is extreme cold, makes it the solid dangerous to handle without protection. DO NOT touch or handle on bare skin.
DRY ICE IS NOT NORMAL ICE (NOT MADE FROM WATER) CAN NOT EAT OR USE WITH ANY KIND OF BEVERAGE AS NORMAL ICE ( WATER ). DRY ICE EXTEMERELY COLD, MAKES IT THE SOLID AND DANGEROUS TO BARE SKIN, THE MOUTH AND ALSO GASTROINTESTINAL TISSUE.
Prolonged exposure to dry ice can cause severe skin damage through frostbite, and the fog produced may also hinder attempts to withdraw from contact in a safe manner. Because it sublimes into large quantities of carbon dioxide gas, which could pose a danger of hypercapnia. Dry ice should only be exposed to open air in a well-ventilated environment.
For this reason, dry ice is assigned the S-phrase S9 in the context of laboratory safety. Industrial dry ice may contain contaminants that make it unsafe for direct contact with foodstuffs. Tiny dry ice pellets used in dry ice blast cleaning do not contain oily residues.
Although dry ice is not classified as a dangerous substance by the European Union or as a hazardous material by the USA, Department of Transportation for ground transportation, when shipped by air or water, it is regulated as a dangerous good and IATA packing instruction 954 (IATA PI 954) requires that it be labeled specially, including a diamond-shaped black-and white label, UN 1845. Also, arrangements must be in place to ensure adequate ventilation so that pressure build-up does not rupture the packaging. The Federal Aviation Administration in the US allows airline passengers to carry up to 2.5 kg (5.5 lb) per person either as Checked baggage or carry-on baggage, when used to refrigerate perishables.
To carry / transport of dry ice required insulated styro container and to handle must wear gloves with different size and insulation thickness
Find attachment : Comparison of phase diagrams of carbon dioxide (red) and water (blue) as a log-lin chart with pressure from 100 Pa to 100 MPa and temperature from 0 K to 650 K, compiled from data in
http://www.lsbu.ac.uk/water/phase.html , http://ergodic.ugr.es/termo/lecciones/water1.html
And Finally,
Nutty Fact:
In 1925, the solid form of CO2 was trademarked by the DryIce Corporation of America as "Dry ice", and than this solid form got its common name – “dry ice”




Post a comment